Concerned about their appeal to children, the FDA wants to ban minty smokes.

The fight against smoking is, by his own admission, deeply personal for Scott Gottlieb, head of the Food and Drug Administration (fda), an American regulatory agency. Having worked as a doctor and survived cancer, he has seen its effects up close and is determined to get Americans to quit tobacco. On November 15th he announced widely expected measures to restrict the sale of flavoured vaping products. More surprising was a proposed ban on menthol cigarettes.
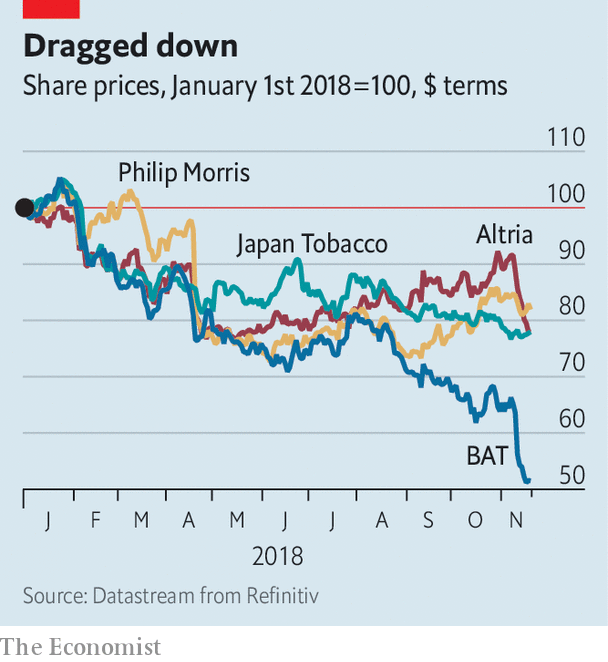
The minty smokes represent 35% of the American tobacco market. In only a few other countries, such as Hong Kong, Singapore and Thailand, are they as popular. Even as overall rates of smoking in America have been declining recently, consumers’ preference for menthol cigarettes has increased slightly. The fda laid out no time frame for its potential ban but its announcement sent big tobacco companies’ share prices plummeting nonetheless. British American Tobacco (bat), the second-largest non-state producer, was worst hit. It owns rj Reynolds, which makes Newports, America’s biggest-selling brand of menthols. Around 55% of Reynolds’s sales in America are menthol cigarettes, according to Wells Fargo, a bank. bat’s shares have fallen by 20% since talk of a ban began. Canada’s federal government banned the menthol variety last year.
In America mint-flavoured cigarettes are particularly popular with two groups: black smokers and young ones. Over 80% of African-American smokers puff on menthol cigarettes, according to national drugs surveys. That is a result of marketing by tobacco companies. Philip Morris, one of the biggest, commissioned research in 1953 that showed that although only 2% of white Americans preferred Kools, a brand of menthol smokes, 5% of black Americans did. This slight difference was then seized upon by advertisers and exploited. Elston Howard, a black baseball player, became a spokesman for Kools. “Papa’s got a brand new bag”, a hit by James Brown, was used to advertise Newports in the late 1970s. Tobacco companies became sponsors of such events as the “Kool Jazz Festival”.
Just over half of all smokers aged between 12 and 17 also plump for menthols, compared with less than a third of those over 35. For young people, the appeal of such cigarettes is explained by menthol’s capacity to mask the flavour of smoke and soothe the irritation that novice smokers often experience. As such, the fda sees them as a dangerously appealing route into smoking. Previous research by the regulator have shown that although there is little evidence to show that menthol cigarettes are more or less toxic than any other kind, smokers appear to be more heavily addicted to them and less likely to give them up.
The agency’s proposals will prompt a fierce pushback from the industry, reckons Dennis Henigan of the Campaign for Tobacco-Free Kids in Washington, dc. He expects that tobacco companies will challenge the argument that menthol cigarettes pose a distinct public-health risk. bat argues that “the science today does not support treating them differently from other cigarettes”. Tobacco companies may also argue that a ban will lead to a boom in illicit sales. The counter-argument is that it is far-fetched to think that any illegal trade would be so extensive as to undercut the public-health gains. Producing mentholated cigarettes illegally on such a scale without being detected would be hard.
Analysts, however, are sceptical that the ban will be enacted any time soon. The tobacco firms’ arguments are convincing, many reckon. The scale of their legal challenge could prevent it. Such a move would go much further than previous efforts to deter Americans from smoking. Cigarette packets do carry written warnings about the dangers of smoking but a law from 2009 mandating graphic health warnings has not yet been implemented.
Still, the pressure on tobacco companies is unlikely to ease. Mr Gottlieb wants to reduce the amount of nicotine permitted in cigarettes, cutting it to non-addictive levels. In future the big firms plan to rely increasingly on new products such as iqos, a “heat-not-burn” smokeless device produced by Philip Morris International but not yet authorised for sale in America. The firm hopes it will become the first tobacco product the fda allows to be advertised as less harmful than cigarettes. But if such innovations do not reduce the number of young people smoking, Mr Gottlieb has made it clear that he will not hesitate to take more aggressive steps.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.